
Research
The close interactions between basic researchers and physicians together with a translational medicine approach are imperative prerequisites for clinical relevant innovations in Oncology and Hematology.
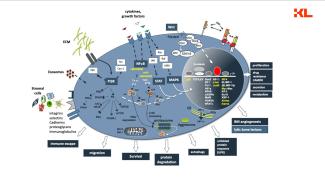
Research projects of the Division of Moleculare Oncology and Hematology
KL/K.Podar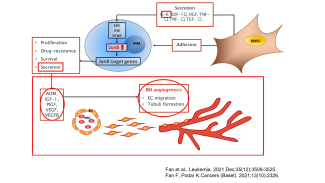
Researchproject Division Moleculare Oncology and Hematology
KL/K.Podar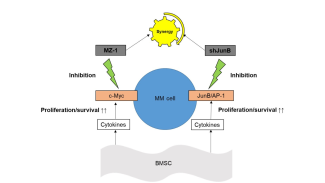
Researchproject Division Moleculare Oncology and Hematology
KL/K.Podar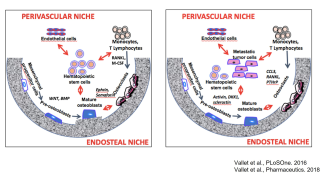
Researchproject Division Moleculare Oncology and Hematology
KL/K.Podar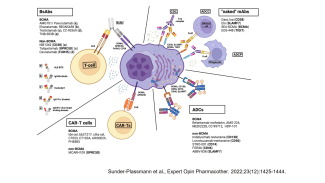
Researchproject Division Moleculare Oncology and Hematology
KL/K.Podar
Researchproject Division Moleculare Oncology and Hematology
KL/K.Podar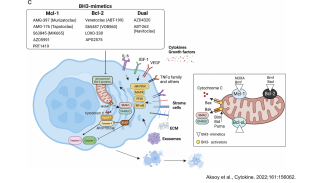
Researchproject Division Moleculare Oncology and Hematology
KL/K.Podar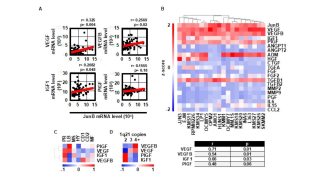
Researchproject Division Moleculare Oncology and Hematology
KL/Klaus Podar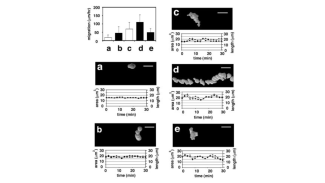
Researchproject Division Moleculare Oncology and Hematology
KL/K.Podar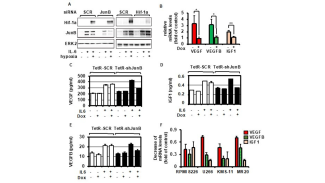
Forschungsprojekt Fachbereich Molekulare Onkologie und Hämatologie
KL/K.Podar
Forschungsprojekt Fachbereich Molekulare Onkologie und Hämatologie
KL/K.Podar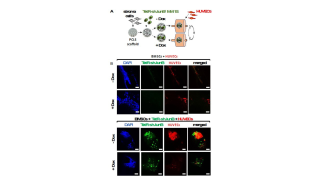
Researchproject Division Moleculare Oncology and Hematology
KL/K.Podar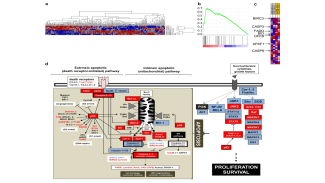
Researchproject Division Moleculare Oncology and Hematology
KL/K.PodarBASIC RESEARCH
In basic research, the Division of Molecular Oncology and Hematology seeks to: (1) decipher molecular processes that contribute to the development and progression of hematologic (e.g., multiple myeloma, myeloproliferative disorders) and solid (e.g., breast cancer, genitourinary carcinomas) malignancies within the tumor microenvironment; (2) to identify novel biomarkers; and (3) to preclinically develop and evaluate novel agents and therapeutic strategies.
- the pathophysiological role of AP-1 family transcription factors in multiple myeloma (Fan et al., Leukemia 2017 and Leukemia 2021) and breast carcinoma.
- the pathophysiological role of the anti-apoptotic Bcl-2 family member Mcl-1 (Bashari et al. Breast Cancer Res 2016; Vallet et al, Breast Cancer Res Treat 2019).
- approaches to therapeutically reactivate the tumor-associated immune system.
- targeted degradation of tumor-associated proteins
- identifying molecular mechanisms that contribute to tumor-associated bone disease (Vallet et al, PLoS One 2016).
CLINICAL RESEARCH:
In addition to basic research projects, the group contributes to clinical trials, which investigate novel treatment regimens for induction and maintenance as well as relapsed/ refractory patients, in multiple myeloma and urothelial carcinoma in particular. For example, we recently contributed to a study demonstrating the anti-myeloma activity of the first-in-class selective nucleus export inhibitor (SINE), selinexor, which led to the approval of this agent in combination with dexamethasone for patients with refractory triple myeloma (Chari et al., N Engl J Med, 2019).
- A phase 3 Study of Elranatamab (PF-06863135) Monotherapy and Elranatamab + Daratumumab Versus Daratumumab + Pomalidomide + Dexamethasone in Participants With Relapsed/Refractory Multiple Myeloma (MAGNETISMM-5) (Ongoing)
- A randomized phase 3 non-inferiority trial assessing lenalidomide, bortezomib and dexamethasone induction therapy with either intravenous or subcutaneous isatuximab in patients with newly diagnosed multiple myeloma (GMMG-HD8 / DSMM-XIX IITTrial)
- Phase 3 Study of Teclistamab in Combination with Lenalidomide versus Lenalidomide Alone in Participants with Newly Diagnosed Multiple Myeloma as Maintenance Therapy Following Autologous Stem Cell Transplantation (MajesTEC-4)
- CA017078: A randomized phase 3 study of neoadjuvant chemotherapy as monotherapy vs. neoadjuvant chemotherapy plus nivolumab or nivolumab and BMS-986205 followed by continued postoperative therapy with nivolumab or nivolumab and BMS-986205 in patients with muscle-invasive bladder cancer (Closed)
- VOLGA (active, recruiting):
A randomized phase 3 study of perioperative therapy with durvalumab in combination with tremelimumab and enfortumab vedotin or durvalumab in combination with enfortumab vedotin in patients with muscle-invasive bladder cancer who are not suitable for cisplatin or refuse cisplatin. - EVOPAR-Prostate01 (Planned start July 2024):
A randomized, double-blind, placebo-controlled phase 3 study of saruparib (AZD5305) in combination with an anti-hormonally active drug in patients with metastatic castration-sensitive prostate cancer. - many more in preparation
- Myeloma study:
Short title: AGMT_MM-2
Title: A randomized Phase II, 2-armed study in transplant ineligible patients with newly diagnosed multiple myeloma (NDMM) comparing Carfilzomib + Thalidomide + dexamethasone (KTd) versus Carfilzomib + Lenalidomide + dexamethasone (KRd) induction therapy with respect to response rates and investigating a Carfilzomib (K) monotherapy maintenance strategy- Status: active, not recruiting
- Start: April 2017 (in Austria)
- Coordinating Investigator: Univ. Prof. Dr. Heinz Ludwig
- EudraCT Nummer: 2016-000475-24
- ClinicalTrialsID: NCT02891811
- Number of patients: 146 (international)
- Sponsor: AGMT gemeinnützige GmbH
- A randomized Phase II, 2-armed study in transplant ineligible patients with newly diagnosed multiple myeloma (NDMM) comparing Carfilzomib + Thalidomide + dexamethasone (KTd) versus Carfilzomib + Lenalidomide + dexamethasone (KRd) induction therapy with respect to response rates and investigating a Carfilzomib (K) monotherapy maintenance strategy
- AGMT
- Klinische Abteilung für Innere Medizin 2
- Klinische Abteilung für Urologie
If you or your physician are interested in these studies, please write to Klaus.podar@krems.lknoe.at or Sonia.vallet@krems.lknoe.at
Research projects of the Division of Molecular Oncology & Hematology
The Division of Molecular Oncology & Hematology was founded in October 2022 and replaced the former scientific working group Molecular Hematology & Oncology. Some of the projects mentioned are therefore still assigned to the scientific working group.
Please visit our research information system KRIS for a comprehensive overview. Below you will find an excerpt of projects.


